CREDIT: SANJUAN NANDIN ET AL., 2017
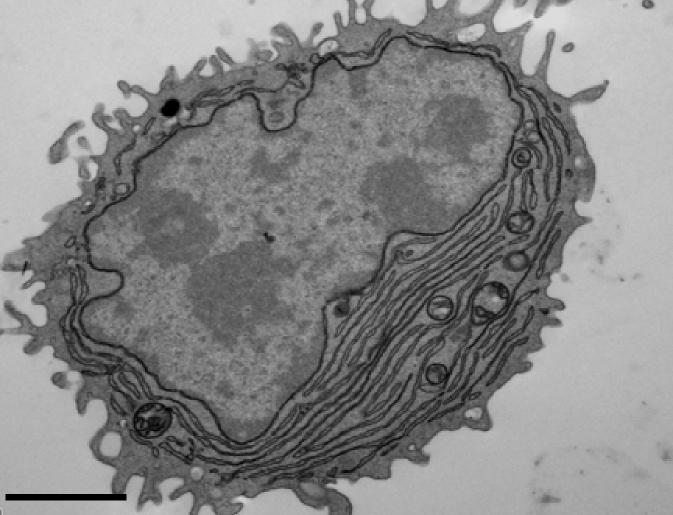
An international team of researchers led by Facundo Batista, from the Francis Crick Institute in London, has a new technique to swiftly manufacture specific human antibodies. Antibodies are large Y-shaped proteins that are enlisted by the immune system to target and eliminate foreign objects such as bacteria and viruses. An antibody produced by the body’s B cells has a single built-in antigen key that helps recognize a specific invading organism. The target antigen (foreign organism) and the antibody have a compatible structure at the tips of their “Y” structures, so when there is binding via the key fitting into the lock, the antibody is triggered, to mark or nullify its target.
In the immune system’s defense, it is up to B cells to identify pathogens, so it can multiply and create plasma cells that discharge an army of antibodies. The study published in The Journal of Experimental Medicine duplicates these steps to spawn specialized antibodies from B cells separated from human blood samples. A major challenge for the diverse research team which included members of the Ragon Institute of MGH, MIT, and Harvard, is that not every B cell can produce a specific antigen response. Also, B cells need a second signal to start the plasma cells proliferation process, which comes from short DNA fragments called CpG oligonucleotides, which activate a protein called TLR9 inside B cells.
In the first attempt to beat this obstacle using blood samples drawn in the laboratory, the team introduced TLR9 from CpG oligonucleotides, which ended up activating all the B cell, instead of just the ones that could target that antigen. To get around these challenges the team took the extracted B cells from blood cells and added tiny nanoparticles coated with both CpG oligonucleotides infusing TLR9 and the pertinent antigen. Only the B cells that recognized the specific antigen used the TLR9 to produce the specific antibodies. The procedure worked in tests using a variety of bacterial and viral antigens, including the tetanus toxoid and several strains of influenza A and is not reliant on the donors having been formerly exposed to any of these antigens via infection or vaccination. The team’s hope is this methodology for producing specific antibodies will be used by other researchers to enhance or create new treatments of infectious diseases and perhaps certain cancers.