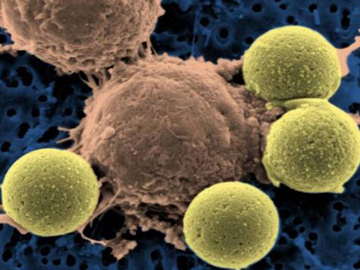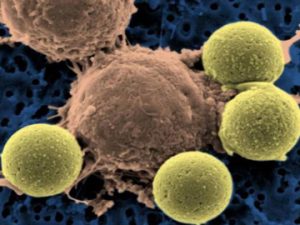
An engineered CAR T cell (center) binding to beads, which cause the T cell to divide.
Credit: University of Pennsylvania Perelman School of Medicine
In a new step forward in medicine, a new targeted cancer treatment that creates genetically engineered cells from a patient’s immune system to search and assail only the bad cancer cells, may be on the market soon. The new treatment approach called CAR-T cell immunotherapy is named CTL019 or tisagenlecleucel, and is designed for children and young adults ( ages 3 to 25), who are fighting a common form of leukemia called B cell acute lymphoblastic leukemia. This blood cancer is the most common childhood cancer in the United States.
The CTL019 drug developed by the University of Pennsylvania and Novartis Corp. does have potentially serious side effects, but it gained a unanimous recommendation from the FDA’s Oncologic Drugs Advisory Committee. While the FDA does not always follow their committees’ recommendations, the overwhelming committee approval could mean the treatment could be approved by the FDA by the end of September. CTL019 will be utilized as a secondary treatment option for those have relapsed after undergoing standard treatment for B cell acute lymphoblastic leukemia. Survival rates for this type of childhood leukemia are high with standard treatment. But when standard treatment fails, survival chances are slim without an option like CAR-T therapy, as other alternatives are even more toxic.
For years, scientists have tried to develop treatments that provoke the immune system to fight cancer, and recently, scientists developed a new generation of “immunotherapy” drugs that target specific forms of cancer and have produced impressive results. This new one-time targeted cancer infusion therapy known as CAR-T cell immunotherapy also works by unleashing the body’s natural defense system. Key immune system cells known as T cells are drawn from the patient and are genetically programmed to seek out and attack only cancer cells. Doctors then infuse millions of the genetically modified revved-up T cells referred to as a “living drug” treatment, back into the patient’s body, theoretically leaving healthy tissue unharmed.